




Our technological reliability has already been proven by several previous toxicity tests and efficacy tests.
This time, we are the first to conduct clinical trials of the PPAR γ inflammation mechanism, which was not implemented not only in Korea but also abroad, with a drug manufactured at a large-capacity production facility of GMP.

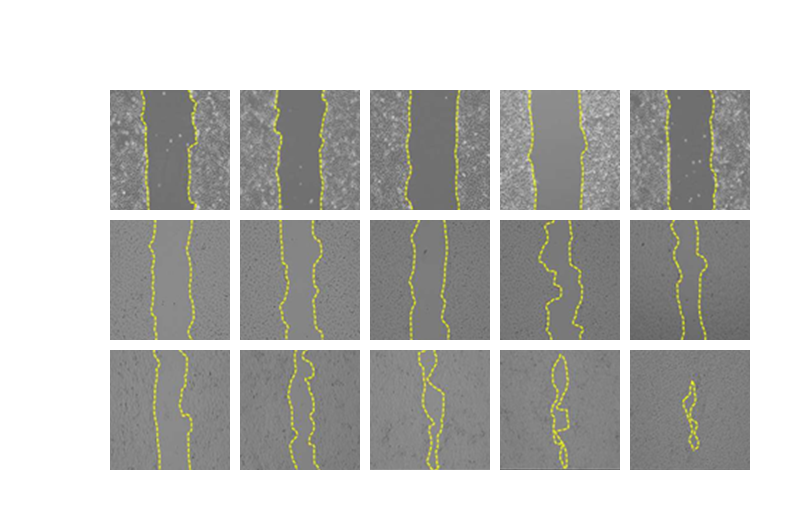
Our technological differentiation is to transform into different plant cell combinations to generate and develop new anti-inflammatory.
The biggest problem in current biological systems of other companies is safety and the transformability of bacteria.
There are risk factors such as the problem of separation in the process of cloning and applying bacteria to clone, and the environment in which bacteria are converted, but our company These challenges have been solved.
targeted therapy

Skin microbiome suppression
inhibition of mRNA expression
PPAR γ inflammation suppression
inflammatory skin disease
biofilm inhibition
LPS control for inflammatory substances